 Discovery Information Discovery Information
|
| Who: H. Davy, J.L. Gay-Lussac, L.J. Thenard |
| When: 1828 |
| Where: England/France |
|
 Name Origin Name Origin
|
| From borax and carbon. |
| "Boron" in different languages. |
|
 Sources Sources
|
| Obtained from kernite, a kind of borax (Na2B4O7.10H2O). The world wide commercial borate deposits are estimated to be 1010 kg of boron.
|
| The USA, Tibet, Chile and Turkey are important producers. Around 1 million tons of boric anhydride (B2O3) are produced each year.
|
|
 Abundance Abundance
|
| Universe: 0.001 ppm (by weight) |
| Sun: 0.002 ppm (by weight) |
| Carbonaceous meteorite: 1.6 ppm |
| Earth's Crust: 950 ppm |
| Seawater: 4.41 ppm |
| Human: |
| 700 ppb by weight |
| 410 ppb by atoms |
|
 Uses Uses
|
| Used with titanium and tungsten to make light weight heat resistant alloys. Also tennis rackets, regulators in nuclear plants, heat resistant glass and eye disinfectant. Boric acid (H3BO3) is used an insectiside, mostly against ants or cockroaches.
|
| Boron nitride is a material in which the extra electron of nitrogen (with respect to carbon) enables it to form structures that are isoelectronic with carbon allotropes.
|
| Sodium tetraborate decahydrate (Na2B4O7 - 10H2O) or borax, used in the production of adhesives, in anti-corrosion systems and many other uses.
|
| Sodium tetraborate pentahydrate (Na2B4O7 - 5H2O), which is used in large amounts in making insulating fiberglass and sodium perborate bleach.
|
| Orthoboric acid (H3BO3) or boric acid is used in the production of textile fiberglass, flat panel displays and eye drops.
|
| Boron slurry is used as an energetic material with very high energy density like rocket fuels and jet engines. |
|
 History History
|
| Compounds of boron have been known of for thousands of years. In early Egypt, mummification depended upon an ore known as natron, which contained borates as well as some other common salts. Borax glazes
were used in China from 300 AD, and boron compounds were used in glassmaking in ancient Rome.
|
| The element was not isolated until 1808 by Sir Humphry Davy, Joseph Louis Gay-Lussac, and Louis Jacques Thenard, to about 50 percent purity, by the reduction of boric acid with sodium or magnesium. These men did not recognize the substance as an element. It was Jöns Jakob Berzelius in 1824 that identified boron as an
element. The first pure boron was produced by the American chemist W. Weintraub in 1909, although this is disputed by some
researchers.
|
|
 Notes Notes
|
| At standard temperatures boron is a poor electrical conductor but is a good conductor at high temperatures. |
| Boron nitride can be used to make materials that are almost as hard as diamond. The nitride also acts as an electrical insulator but conducts heat similar to a metal. This element also has lubricating
qualities that are similar to graphite.
|
| Boron is never found in the elemental form in nature. It was first obtained by Moissan in 1895 by reduction of boric anhydride with magnesium in a thermite-type reaction. This is still used for obtaining large quantities of impure boron. Highly purified crystalline
boron is obtained by vapour phase reduction of the compound boron trichloride with hydrogen on electrically heated filaments in a flow system.
|
| The United States and Turkey are the world's largest producers of boron.
|
|
 Hazards Hazards
|
| Elemental boron and borates are not toxic and therefore do not require special precautions while handling. Some of the more
exotic boron hydrogen compounds, however, are toxic as well as highly flammable and do require special handling care.
|
| Boron is highly flammable. |

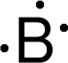

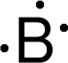
 Discovery Information
Discovery Information
 Name Origin
Name Origin
 Sources
Sources
 Abundance
Abundance
 Uses
Uses
 History
History
 Notes
Notes
 Hazards
Hazards